Small Vessel Diseases: Starting Point for Major Diseases
The diseases of the small vessels are the starting point for major diseases such as high blood pressure, diabetes, strokes, heart attacks, dementia, and other brain-related diseases
Reference: “Small Blood Vessels: Big Health Problems?” Scientific Recommendations of the National Institutes of Health Workshop, Francesca Bosetti et al., American Heart Association, 2016 Nov 4;5(11):e004389. doi: 10.1161/JAHA.116.004389.
Introduction
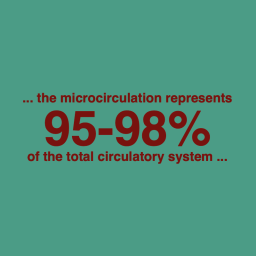
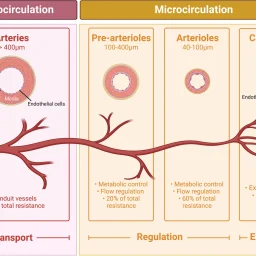
Small blood vessels (generally <100 μm in internal diameter) contribute to fundamental physiological processes and pathological events, but may not necessarily garner the attention associated with macrovascular physiology and disease. The complexity and widespread distribution of small vessels contribute to a bench-to-bedside research gap.
Understanding the structural and functional signatures of small vessels and how local perturbations contribute to systemic pathophysiological conditions has the potential to transform diagnostic and therapeutic approaches.
The NIH held a workshop on September 18–19, 2014, bringing together scientists and clinicians from diverse areas of microvascular research to share discoveries, identify challenges, and foster collaborative research. Sessions included: Basic Biology, Vascular Dynamics, Small Vessel Cellular Interactions, Transendothelial Transport, Small Vessels in Disease, Effects of Internal Milieu, and Research Tools and Innovation. All sessions are available at the NIH Videocast archive: http://videocast.nih.gov/PastEvents.asp. Top priorities are summarized in Table 1.
| Priority | Description |
|---|---|
| Basic biology and natural history of small vessels | Identify therapeutic targets considering heterogeneity in structure and function, genetic determinants, sex, hormones, and age. |
| Vascular dynamics | Visualize subcellular signaling networks, intermolecular interactions, and cell–matrix properties in health and disease. |
| Small vessel cellular interactions | Understand molecular and cellular processes and how organ-specific environments influence vessel response. |
| Transendothelial transport (including BBB) | Analyze regulation and function of the neurovascular unit in health and disease. |
| Small vessels in disease | Develop mechanism-based therapies to prevent or slow small vessel disease progression and define treatment strategies. |
| Effects of internal milieu and disease | Develop clinically relevant models and study interactions between vasculature, inflammation, and immune activation. |
| Research tools and innovation | Integrate biological, technological, and computational advances to understand dynamic signaling interactions in micro- and macrovascular networks. |
Basic Biology and Natural History of Small Vessels
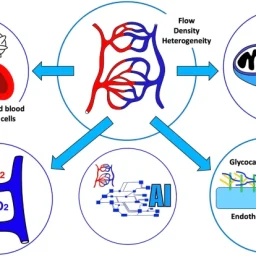
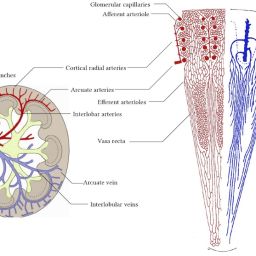
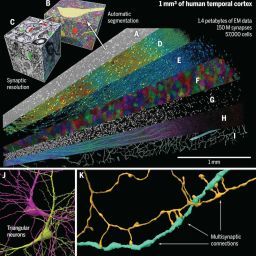
The common structural component of all small blood and lymphatic vessels is the endothelium. Capillaries are the simplest vessels with the smallest diameter. Pericytes are abundant on arterioles and venules, but sparse on capillaries outside the brain.1 Endothelial heterogeneity is evident at the capillary level and varies between organs. Functions include nutrient exchange, signaling, immune function, antithrombotic surface maintenance, and vasomotor tone regulation.4

Figure 1: Differences in the functional architecture of small blood vessels across mouse organs. Fluorescent microspheres demonstrate variations in endothelium permeability and perfusion. Source: Zorina Galis, unpublished data.
Core source: https://pubmed.ncbi.nlm.nih.gov/27815267/
Original source, Journal of the American Heart Association: https://www.ahajournals.org/doi/10.1161/JAHA.116.004389
Frequently Asked Questions
What are small vessel diseases?
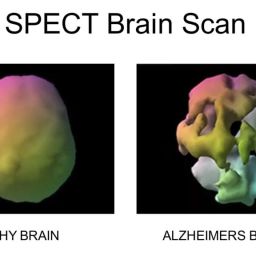
Small vessel diseases affect tiny blood vessels less than 100 micrometers in diameter, impacting organs like the brain, heart, kidneys, and contributing to major health problems.
Why are small vessels important?
They control microcirculation, nutrient exchange, immune response, and are critical in the development and progression of chronic diseases like diabetes, hypertension, and dementia.
Where can I access NIH workshop videos?
NIH workshop videos are available at http://videocast.nih.gov/PastEvents.asp.