Microcirculatory Dysfunction and Resuscitation: Why, When, and How
Authors: J.P.R. Moore, A. Dyson, M. Singer, J. Fraser – Open Archive DOI:
https://doi.org/10.1093/bja/aev163
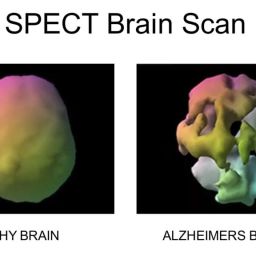
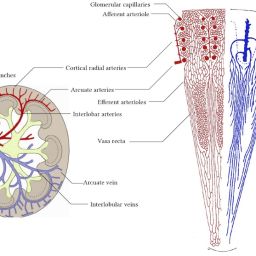
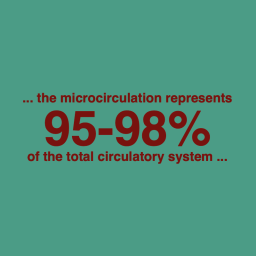
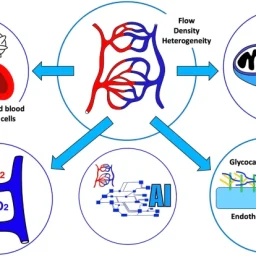
The study explores the role of microcirculation and its endothelium as a central organ system. Microvessels smaller than 150 µm—including arterioles, venules, and capillaries—determine oxygen delivery and tissue health. Unique architectures in the kidney, gut, and liver reflect functional requirements.
Blood flow regulation relies on myogenic, metabolic, and neurohumoral mechanisms. These control vascular tone, ensure oxygen delivery, and adapt to tissue demands. Dysfunctions in shock—hypovolemic, cardiogenic, or septic—disrupt perfusion and oxygenation, leading to organ failure.

Microcirculation’s Role in Oxygen Delivery
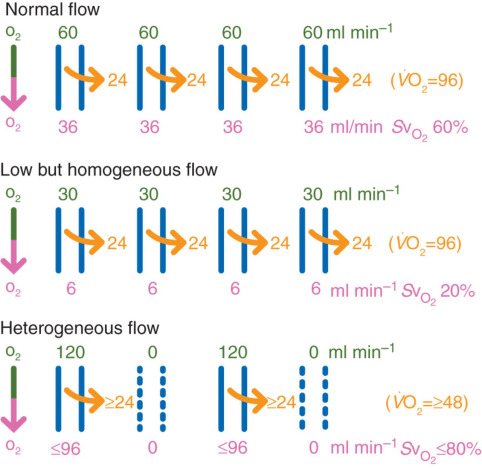
In health, microcirculation maintains oxygen supply despite systemic changes. In shock, microvascular collapse causes tissue hypoxia. Early resuscitation can restore balance, but monitoring tools are still evolving. Focusing only on blood pressure or cardiac output may miss underlying microvascular failure.
Read the full study:
https://www.bjanaesthesia.org/article/S0007-0912(17)31149-2/fulltext